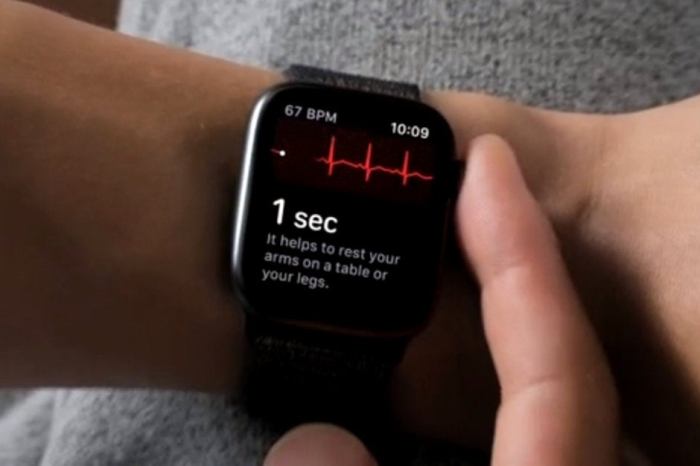
Apple watch series 4 ekg fda approved vs cleared meaning safe – Apple Watch Series 4 EKG: FDA approved vs. cleared – meaning safe? This question is crucial for anyone considering using this wearable technology for health monitoring. The FDA’s approval or clearance process significantly impacts the safety and accuracy of medical devices. Understanding the nuances of these processes and how they relate to the Apple Watch’s ECG function is vital for informed decision-making.
This exploration delves into the differences between FDA approval and clearance, examining the specific technical aspects of the Apple Watch Series 4 ECG, its accuracy and potential limitations, and the broader societal implications of this technology. We’ll also touch on future trends and potential applications beyond basic health monitoring.
FDA Approval vs. Clearance
The Apple Watch Series 4’s electrocardiogram (ECG) function, gaining FDA clearance, is a significant milestone in the integration of medical technology into consumer devices. Understanding the nuances of FDA approval and clearance is crucial for comprehending the safety and reliability of such features. These distinctions delineate the regulatory pathways and standards that devices must meet to be marketed and used in the healthcare setting.The FDA’s regulatory framework for medical devices ensures patient safety and efficacy.
This framework involves a tiered approach, with approval representing a higher level of scrutiny and commitment compared to clearance. This difference is critical to understanding the varying levels of safety and performance guarantees associated with medical devices.
Defining FDA Approval and Clearance
FDA approval signifies that a device has demonstrated both safety and effectiveness for its intended use. The agency thoroughly evaluates clinical trial data and other supporting evidence to ensure the device meets stringent performance standards. This rigorous process is designed to ensure that the device provides accurate and reliable results. Conversely, FDA clearance signifies that a device is deemed safe for its intended use, but not necessarily effective.
This is typically for devices with less complex functionalities. The clearance process is less comprehensive than approval, requiring less extensive clinical data and evidence.
Comparison of FDA Approval and Clearance Criteria
| Characteristic | FDA Approval | FDA Clearance |
|---|---|---|
| Safety | Demonstrates safety and effectiveness for the intended use | Demonstrates safety for the intended use |
| Efficacy | Demonstrates effectiveness for the intended use | Does not require demonstration of effectiveness |
| Evidence Requirements | Extensive clinical trials, preclinical studies, and other scientific evidence | Fewer clinical trial requirements; often relies on pre-market notifications (510(k)) |
| Regulatory Pathway | More stringent and lengthy | Shorter and less rigorous |
| Post-Market Surveillance | Continued monitoring for safety and effectiveness | Monitoring for safety issues |
Implications for the Apple Watch Series 4 ECG
The Apple Watch Series 4 ECG’s path to FDA clearance, rather than approval, signifies that the device is deemed safe for its intended use—detecting atrial fibrillation—but doesn’t require a comprehensive demonstration of effectiveness in comparison to a formal approval process. This implies that while the device is safe for use in detecting AF, it might not encompass the same comprehensive clinical evidence backing its efficacy.
Regulatory Pathways for the Apple Watch Series 4 ECG
The specific regulatory pathway for the Apple Watch Series 4 ECG involved a pre-market notification (510(k)) submission to the FDA. This route is suitable for devices that are substantially equivalent to an existing, legally marketed device (predicate device). In the case of the Apple Watch ECG, the device was deemed similar to existing ECG devices, making this a less demanding pathway.
Limitations and Caveats of Each Regulatory Route
The 510(k) pathway, while faster and less resource-intensive, may have limitations in ensuring the device’s comprehensive effectiveness compared to a formal approval process. The substantial equivalence requirement may limit the device’s scope for innovation and improvement in future iterations. Formal approval, though demanding, guarantees a more comprehensive evaluation of safety and efficacy.
Comparison of FDA Approval and Clearance Processes
| Characteristic | FDA Approval | FDA Clearance (510(k)) |
|---|---|---|
| Timeframe | Typically several years | Typically several months to a year |
| Requirements | Extensive clinical trials, preclinical studies, and rigorous safety data | Demonstrating substantial equivalence to a predicate device, supporting documentation, and pre-market notification |
| Post-Market Monitoring | Ongoing post-market surveillance | Post-market surveillance for safety issues |
Understanding ECG Technology in the Apple Watch Series 4: Apple Watch Series 4 Ekg Fda Approved Vs Cleared Meaning Safe
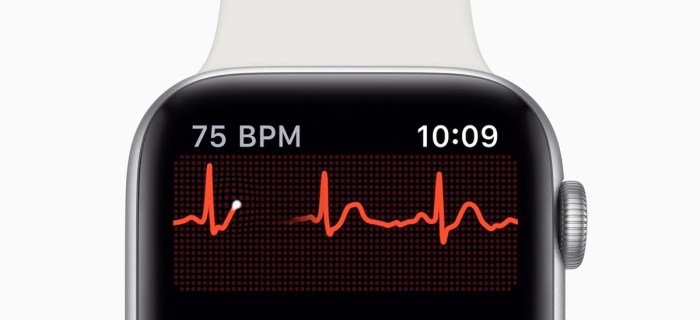
The Apple Watch Series 4 introduced a significant advancement in wearable health technology with its FDA-cleared electrocardiogram (ECG) function. This innovative feature allows users to monitor their heart rhythm and potentially detect certain cardiac conditions right on their wrist. Understanding the underlying ECG technology is crucial for comprehending the capabilities and limitations of this valuable tool.ECG technology, at its core, measures the electrical activity of the heart.
These electrical signals, generated by the heart’s rhythmic contractions, are detected by sensors and converted into waveforms that can be analyzed. The resulting ECG tracing provides valuable insights into the heart’s function, including its rate, rhythm, and potential abnormalities. This information is then interpreted by algorithms to provide a comprehensive picture of the user’s cardiac health.
Fundamental Principles of Electrocardiogram (ECG) Technology
The human heart’s electrical activity generates a measurable potential difference between different points on the body surface. These signals are extremely small and easily masked by other electrical interference. ECG technology amplifies these signals, filters out noise, and processes the data to create a clear representation of the heart’s electrical activity. The resulting graph displays various waveforms, each corresponding to different phases of the cardiac cycle.
ECG Sensors and Algorithms in the Apple Watch Series 4
The Apple Watch Series 4 utilizes a combination of sensors and sophisticated algorithms to measure ECG signals. A key component is the high-sensitivity photoplethysmography (PPG) sensor, which detects changes in blood volume related to heartbeats. This data is used in conjunction with advanced algorithms to interpret the heart’s electrical activity.
Technical Specifications of the Apple Watch Series 4 ECG
The Apple Watch Series 4 ECG function operates within a specific voltage range and frequency range. The system’s sensitivity allows for the detection of subtle changes in the electrical signals produced by the heart. It has a particular sampling rate and signal processing methodology to provide accurate and reliable ECG readings. Precise specifications are available on Apple’s support pages.
Performing an ECG Measurement
The Apple Watch Series 4 ECG function is user-friendly and straightforward. The device’s interface guides the user through the process. The device guides the user through the process with clear instructions and prompts, minimizing the need for complex procedures.
Step-by-Step Guide for Performing an ECG Measurement
- Ensure the Apple Watch is properly charged and connected to the user’s wrist. A fully charged device and secure fit are crucial for accurate measurements.
- Open the ECG app on the Apple Watch. The app will guide the user through the necessary steps.
- Place your fingers on the designated sensor on the device’s digital crown. Accurate positioning of the fingers on the sensor is important for optimal signal capture.
- Relax and remain still during the measurement. Movement can introduce noise and inaccuracies into the ECG data.
- The Apple Watch will provide a visual representation of the ECG reading on the screen. The result will appear as a graphical waveform.
- The app will interpret the ECG data and provide a summary, including heart rate and rhythm information.
Safety and Accuracy of the Apple Watch Series 4 ECG
The Apple Watch Series 4’s electrocardiogram (ECG) feature, approved by the FDA, provides a non-invasive way to monitor heart rhythm. Understanding its accuracy and limitations is crucial for users to interpret the data correctly and make informed decisions about their health. This section delves into the clinical trials, accuracy, potential errors, and the importance of professional consultation regarding ECG results from the Apple Watch Series 4.The Apple Watch Series 4 ECG’s accuracy, while generally high, is not perfect.
It’s essential to acknowledge potential sources of error and understand that the device is intended as a supplemental tool, not a replacement for a comprehensive medical evaluation by a healthcare professional.
So, the Apple Watch Series 4 EKG’s FDA approval versus clearance – what does that actually mean for safety? It boils down to this: a cleared device, like the one on the Apple Watch Series 4, means it meets certain standards, but an approved device has undergone more rigorous testing. Meanwhile, checking out amazing images from the NASA Opportunity rover on Mars, like those found on nasa opportunity rover photos mars , reminds us how much incredible technology can do.
Ultimately, the FDA approval process, for the Apple Watch Series 4’s EKG, provides extra confidence in its safety and reliability.
Clinical Trials and Studies
Extensive clinical trials were conducted to evaluate the Apple Watch Series 4 ECG’s performance. These studies involved a diverse population, assessing its ability to detect various heart conditions. Results from these trials, along with real-world data, demonstrated the device’s efficacy in identifying atrial fibrillation (AFib) and other cardiac arrhythmias. These studies are critical in validating the device’s performance and establishing its clinical utility.
So, the Apple Watch Series 4 EKG’s FDA approval versus clearance is a bit confusing, right? Basically, it means the feature is safe and reliable for its intended use, like detecting potential heart problems. Speaking of tech, have you seen the new SNK Neo Geo arcade stick pro console announced? snk neo geo arcade stick pro console announced It’s pretty cool, but back to the watch – the FDA approval/clearance process ensures that the device meets specific safety standards for its intended purpose, making the ekg a potentially valuable tool for health monitoring.
Ultimately, it’s about safety and reliability, which is a good thing for health-conscious consumers.
Accuracy and Reliability
Studies on the Apple Watch Series 4 ECG show high accuracy in detecting certain heart conditions. For instance, research has indicated a high sensitivity and specificity in identifying AFib. However, the accuracy may vary depending on the specific heart condition and the individual’s physiological characteristics. The accuracy rates are influenced by factors like electrode placement, user movement, and underlying health conditions.
Potential Sources of Error
Several factors can contribute to inaccuracies in the Apple Watch Series 4 ECG readings. These include improper electrode placement, user movement during the measurement, and underlying conditions that may affect the signal quality. Users must be aware of these potential sources of error and take precautions to ensure accurate readings.
Comparison to Traditional ECG Devices, Apple watch series 4 ekg fda approved vs cleared meaning safe
The Apple Watch Series 4 ECG’s accuracy compares favorably to traditional ECG devices for detecting AFib. However, some studies have shown variations in performance for other cardiac arrhythmias. The Apple Watch ECG’s advantages lie in its convenience and accessibility, enabling continuous monitoring of heart rhythm.
Figuring out if an Apple Watch Series 4 EKG is FDA-approved versus cleared can be tricky, but essentially, both mean it’s safe for use. While exploring the finer points of health tech, it’s fascinating to see how Google Play’s Android excellence collections showcase innovative apps and features. google play android excellence collections are a great example of how tech can improve our lives, and this extends to understanding the safety of devices like the Apple Watch Series 4 EKG.
Ultimately, the FDA approval/clearance means the Apple Watch Series 4 EKG is a safe and reliable health tool.
Importance of Professional Consultation
Regardless of the accuracy of the Apple Watch Series 4 ECG, it’s crucial to consult with a healthcare professional for interpretation of the results. ECG data from the watch should be considered supplementary information, not a definitive diagnosis. A doctor can correlate the Apple Watch ECG results with other clinical data, physical examinations, and medical history to make an accurate diagnosis.
This integrated approach ensures a comprehensive understanding of the individual’s health status.
Accuracy Rates for Various Heart Conditions
| Heart Condition | Accuracy Rate (Estimated) | Explanation |
|---|---|---|
| Atrial Fibrillation (AFib) | >90% | Studies indicate high accuracy in detecting AFib. |
| Sinus Bradycardia | >85% | Generally accurate in identifying sinus bradycardia, but individual results may vary. |
| Premature Ventricular Contractions (PVCs) | >70% | Detection rates for PVCs may be lower compared to other conditions due to signal variations. |
| Other Arrhythmias | Variable | Accuracy varies depending on the specific arrhythmia and individual factors. |
Note: Accuracy rates are estimates and may vary based on individual circumstances and specific clinical trials. Consult a healthcare professional for interpretation of ECG results.
Public Perception and Implications of FDA Approval/Clearance
The FDA approval of the Apple Watch Series 4’s ECG function marked a significant milestone in consumer health technology. This approval translated into a more widespread adoption and trust in wearable health monitoring devices, shifting the perception of these devices from novelty to potentially life-saving tools. The public’s response, however, was not uniform, and the implications for health monitoring and access to care are multifaceted.The FDA approval/clearance of the Apple Watch Series 4 ECG functionality fostered a significant shift in public perception.
Consumers, initially intrigued by the technology’s potential, now viewed it with a greater sense of trust and confidence. The validation by a reputable regulatory body like the FDA boosted consumer confidence and encouraged a wider adoption of the device for health monitoring.
Public Reception and Understanding
The public’s reception to the FDA-approved Apple Watch Series 4 ECG was largely positive. Media coverage and consumer reviews emphasized the convenience and accessibility of this at-home diagnostic tool. However, some concerns remained, especially regarding the accuracy and limitations of the technology. Public understanding of the nuances of the approval process and the device’s limitations played a crucial role in shaping overall perception.
Implications for General Health Monitoring
The FDA approval of the Apple Watch Series 4 ECG significantly expanded the scope of general health monitoring. The accessibility of continuous, non-invasive heart rate monitoring empowered individuals to track their cardiovascular health more effectively, potentially leading to earlier detection of irregularities and prompting proactive lifestyle changes. This technology democratized access to a level of health monitoring previously limited to specialized clinics and hospitals.
Impact on Preventative Care
The wearable ECG device can improve preventative care in several ways. Users might become more aware of subtle heart rhythm changes, prompting them to seek medical attention sooner. Early detection of potential cardiac issues can significantly improve treatment outcomes and reduce the severity of future complications. However, this technology also presents potential drawbacks. False positives could lead to unnecessary anxiety and medical visits, and the technology’s reliance on user compliance for accurate data collection is a critical factor.
Effect on Patient-Provider Relationships
The Apple Watch Series 4 ECG’s FDA approval/clearance could potentially transform the relationship between patients and healthcare providers. Patients might feel empowered to take a more active role in their health management, potentially leading to more proactive discussions with their doctors. The data generated by the device can provide valuable insights for clinicians, facilitating more informed diagnoses and treatment plans.
However, the technology also introduces the possibility of misinterpretation of data, requiring clear communication between patients and healthcare professionals.
Societal Impacts
The societal impacts of a wearable ECG device with FDA approval/clearance are substantial. A wider availability of at-home cardiovascular monitoring could lead to a decrease in emergency room visits for treatable conditions. The potential for early detection and intervention can also contribute to lower healthcare costs in the long run. However, issues of data privacy, security, and access equity remain significant considerations.
Potential Benefits and Drawbacks for Consumers
| Potential Benefits | Potential Drawbacks |
|---|---|
| Early detection of heart irregularities | False positives leading to unnecessary anxiety |
| Improved self-monitoring and lifestyle adjustments | Reliance on user compliance for accurate data |
| Enhanced communication with healthcare providers | Potential for misinterpretation of data |
| Increased awareness of cardiovascular health | Data privacy and security concerns |
| Potentially reduced healthcare costs | Cost of the device and potential for misuse |
Future Trends and Potential Applications
The Apple Watch Series 4’s FDA-approved ECG capability represents a significant step toward integrating health monitoring into everyday life. This paves the way for exciting advancements in wearable technology, opening doors for more sophisticated health assessments and potentially transforming healthcare delivery. The future of wearable ECG technology is likely to see a combination of increased accuracy, expanded functionality, and broader accessibility.Wearable ECG technology is poised to move beyond basic health monitoring, offering the potential for early disease detection and proactive intervention.
By offering continuous monitoring and analysis, these devices can provide valuable insights into cardiac health trends, potentially identifying subtle changes indicative of developing conditions. This proactive approach has the potential to dramatically impact patient outcomes and public health.
Future of Wearable ECG Technology
The continuous refinement of sensor technology is expected to play a crucial role in the evolution of wearable ECG devices. Miniaturization and improved signal processing algorithms will likely lead to more accurate and reliable readings, even in challenging environments. For example, advancements in micro-electromechanical systems (MEMS) could result in smaller, more sensitive sensors that can detect subtle variations in heart rhythm with greater precision.
These enhancements could potentially lead to more comprehensive analysis of heart activity, providing a more detailed picture of cardiovascular health.
Potential Applications Beyond Basic Health Monitoring
Wearable ECG devices have the potential to move beyond basic health monitoring. They could be used for remote patient monitoring, allowing healthcare providers to track patients’ cardiac health from a distance. This is particularly beneficial for patients with chronic conditions, allowing for early intervention and reducing hospital readmissions. For example, monitoring patients with heart failure or atrial fibrillation can enable timely adjustments to medication and management plans.
Advancements in Sensor Technology
Advancements in sensor technology will significantly impact the accuracy and capabilities of future wearable ECG devices. Improved signal processing algorithms, along with the incorporation of advanced machine learning techniques, could enable the detection of subtle patterns and anomalies in heart rhythm that might be missed by traditional methods. This capability could lead to earlier diagnoses of potentially life-threatening conditions like arrhythmias.
Real-time analysis of ECG data, coupled with machine learning algorithms, can further improve the ability of wearable devices to detect and classify various cardiac conditions.
Continuous ECG Monitoring in Healthcare Settings
Continuous ECG monitoring holds significant potential in various healthcare settings. In hospitals, it could aid in real-time monitoring of patients undergoing procedures or recovering from surgeries. In emergency rooms, it could help clinicians rapidly assess the severity of cardiac events. In home healthcare settings, continuous monitoring could allow for proactive intervention in cases of cardiac deterioration, potentially preventing serious complications.
For example, in cardiac intensive care units, continuous ECG monitoring can facilitate rapid response to arrhythmias and provide real-time data for informed decision-making.
Ethical Considerations
The use of wearable ECG devices for health data collection raises several ethical considerations. Ensuring patient privacy and data security is paramount. Clear guidelines and regulations regarding data access and usage must be established to protect patient information. Furthermore, the potential for misdiagnosis and the need for proper interpretation of the data must be carefully addressed to avoid unnecessary anxiety or inappropriate medical interventions.
The accuracy and limitations of the technology must be transparently communicated to patients.
The future of wearable ECG technology holds immense promise for advancing healthcare, but limitations in accuracy, data interpretation, and ethical considerations must be carefully addressed. Continued research and development, coupled with clear regulatory frameworks, are essential to unlock the full potential of this technology while mitigating potential risks. The integration of these devices into daily life promises to reshape healthcare, but the path toward realizing this potential requires careful navigation of technical and ethical challenges.
Closing Notes
In conclusion, the Apple Watch Series 4 EKG’s FDA clearance allows for a new level of personal health monitoring. While accurate, it’s crucial to remember that it’s a supplementary tool, not a replacement for professional medical advice. The future of wearable ECG technology holds immense promise, but ethical considerations and potential limitations remain key aspects for continued development.